Abstract
Background: Genomically-targeted Diffuse Large B-cell (DLBCL) treatments for refractory or relapsing patients remain a clinical need alongside the growing success of Chimeric Antigen T-cells (CAR-T), which have ushered in durable responses in 40-70% of patients. However, recent studies have identified factors indicative of an inferior CAR-T response. These include a "cold" immune microenvironment, downregulation of extrinsic cell death factors such as TNFRSF10B, and alterations to the TP53 tumor suppressor. Few precision treatments exist capable of addressing the consequences, although two avenues are being pursued in clinical trials for lymphoma: inhibition of the TP53 negative regulator MDM2 via Idasnutlin and TP53 reactivation via the unique molecule Eprenetapopt (APR-246). Herein, we report the first observations of synergy between Idasnutlin and Eprenetapopt within DLBCL cell lines and data supporting that TP53 restoration may revive extrinsic apoptosis and immunological pathways capable of modulating CAR-T success.
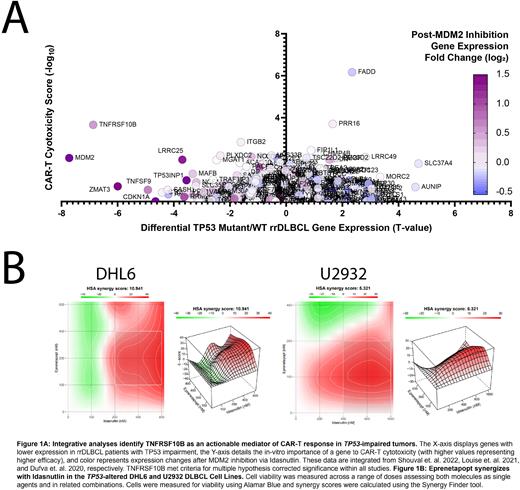
Methods: We analyzed 2 de-novo DLBCL patient cohorts (Xu-Monette et. al. 2020 and the TCGA) for differential expression and pathway analysis between TP53-mutant and Low TNFRSF10B (-1 Standard Deviation) patients versus normal counterparts. We integrated data from 3 additional sources to uncover actionable vulnerabilities within patients faced with dismal CAR-T response: differential gene expression between TP53-mutant and WT rrDLBCL patients (Shouval et. al. 2022), in-vitro CAR-T sgRNA-cytotoxicity scores (Dufva et. al. 2020), and gene expression following MDM2 inhibition (Louise et. al. 2021). We next assayed Idasnutlin and Eprenetapopt vs. 7 DLBCL cell lines, 5 of which harbor TP53 alterations, across a series of 9 concentrations (78nM-20µM) across 3-9 replicates. We followed these results by combining treatments in the TP53-impaired DHL6 and U2932 cell lines, analyzing results using the HSA synergy model. Lastly, we performed Western blot analysis to measure protein expression of TNFRSF10B following 24-hour single-agent or combination treatment.
Results: Integrative analyses support that DNA alterations to TP53 are associated with significantly reduced T-cell activation and extrinsic apoptosis genes. TNFSRF10B was significantly reduced in these cases as well (FDR = 0.0034), and genes with significantly reduced expression in both TP53-mut and Low TNFRSF10B expression were associated with the loss of Immune System Development genes (FDR = 3.66E-09). The Staudt lab IFNG-Up and STAT-Up gene signature pathways were significantly reduced in TP53-mut patients (P < 0.0001, P = 0.483) and in Low TNFRSF10B Patients (P < 0.0001, P = 0.488). TNFRSF10B was also the only gene with significantly reduced expression in TP53-mut rrDLBCL patients, importance for CAR-T cytotoxicity, and increased expression following MDM2 inhibition. Our Western blot results support these observations, showcasing upregulation of TNFRS10B following treatment. Idasnutlin and Eprenetapopt assays vs. DLBCL cell lines demonstrated dose and TP53-dependent cell inhibition, with ED50 values ranging between 880nM to 19.6µM, independent of COO. The homozygous-impaired TP53 cell line Karpas-422 was the least sensitive. The molecules were next combined vs. DHL6 and U2932 cell lines, resulting in synergistic reduction of cell viability and HSA synergy scores of 10.94, and 5.32, respectively. Combined treatments of 400 nM Idasnutlin and Eprenetapopt reduced cell viability significantly greater than single agent treatments at the same concentration in DHL6 (P = 0.0001; P = 0.0176) and U2932 (P = 0.0012; P = 0.0322).
Conclusions: Our results suggest the presence of an interconnected genomic DLBCL phenotype characterized by TP53 loss, reduced TNFRSF10B expression, and a debilitated immune presence, collectively predictive of an inferior CD19 CAR-T response. We applied the targeted therapies Eprenetapopt and Idasnutlin vs. cell line models, with both displaying anti-tumor activity as single agents. Most importantly, we observed synergistic activity in TP53-imparied DLBCL cell lines when the molecules were combined. These results are buoyed by integrative and Western blot analyses that highlight the promise of TNFRSF10B revival afforded by these agents, revealing a potential avenue to address inferior CAR-T response rates faced by patients harboring TP53 alterations.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal